Study Background
IMR-687 is an inhibitor of phosphodiesterase 9 intended to treat sickle cell disease (SCD) by stimulating the production of fetal hemoglobin (HbF). IMR-SCD-102-EXT is an open-label extension (OLE) study of an ongoing Ph 2a, randomized, double-blind, placebo-controlled study (IMR-SCD-102) of IMR-687 in patients with SCD (homozygous sickle hemoglobin [HbSS] or sickle-β0 thalassemia). The Ph 2a study consists of a monotherapy sub-study (IMR-687 vs. placebo) of 6 months and a combination sub-study (IMR-687 + HU vs. HU + placebo) of 4 months. The OLE study examines the long-term benefit and safety of IMR-687 administered for up to 4 years.
Patient Background
Patient 1, a 28-year-old female diagnosed with HbSS, received IMR-687 100 mg once daily (qd) for 3 months, then escalated to 200 mg qd in the monotherapy Ph 2a sub-study. After a 1-month safety follow-up period, the patient enrolled in the OLE, received IMR-687 100 mg qd for an additional 12 months; and subsequently escalated to 200 mg qd per protocol amendment.
Patient 2, a 33-year-old female diagnosed with HbSS, received placebo for 4 months in the combination Ph 2a sub-study. After a 14-month hiatus, the patient enrolled in the OLE, received IMR-687 for ~6 months: 100 mg qd for 4 months, then escalated to 200 mg qd for ~2 months. The patient was on a stable dose of HU for >18 months prior to OLE study and has remained on that same dose during the OLE.
Analysis Methods
Retrospective review of patient medical records allows for comparison of equal time periods prior to and after the start of IMR-687 treatment, with durations of 18 months for Patient 1 and 6 months for Patient 2. For patient-reported outcomes (PROs) and biomarkers, values were compared for the patient's most recent OLE visit and their baseline visit before the first dose of IMR-687, i.e., the start of the Ph 2a study for Patient 1 and the start of the OLE for Patient 2.
Clinical Outcomes: VOCs, Healthcare Use, and PROs
Patient 1 had a 55% reduction in reported vaso-occlusive crises (VOCs) when comparing the 18 months prior to IMR-687 initiation to the 18 months while on IMR-687 (38 to 17 VOCs). Specifically, VOCs that resulted in emergency department (ED) and outpatient visits decreased by 55% (22 to 10 visits) and 50% (14 to 7 visits), respectively, in the periods before versus after IMR-687 initiation. VOCs that resulted in hospitalization decreased from 2 to 0 while on IMR-687.
Analysis of the 18-month period on IMR-687 showed a decrease in reported VOCs with increased time on therapy. In the first 6-month interval, the patient had 9 VOCs, of which 5 were ED visits and 4 were outpatient visits. In the second interval, the patient had 6 VOCs, with 5 ED visits and 1 outpatient visit. In the third and most recent 6-month interval, the patient had 2 VOCs, with 0 ED visits and 2 outpatient visits.
Patient 1 also showed improvement in 5 of the 7 domains (including pain episode frequency and severity) of the Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me®) after 18 months of IMR-687 treatment compared with pre-treatment baseline.
Patient 2 has had no reported VOCs since starting IMR-687, compared with 15 VOCs (all outpatient visits) in the 6 months prior to first dose of IMR-687. The patient also showed a substantial improvement in an average pain score while on IMR-687, from a pre-treatment score of 8/10 to 2/10 after ~6 months of IMR-687 treatment and had a decrease in breakthrough opioid use (morphine sulfate, immediate release) from 63 to 5 tablets weekly.
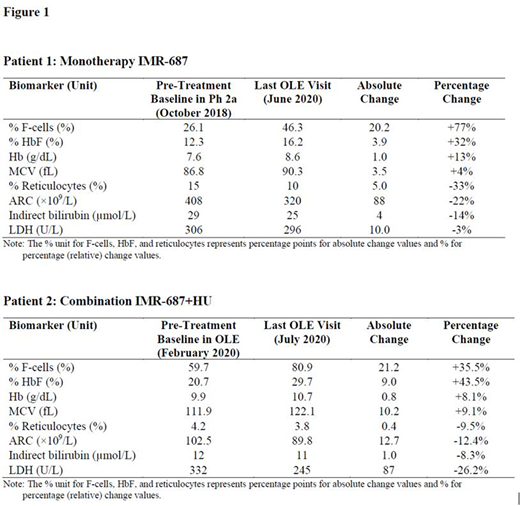
Safety and Biomarker Results
IMR-687 was well-tolerated in both patients. Improvement across key SCD biomarkers was observed for both patients (Figure 1). Parameters included cells positive for fetal hemoglobin (F-cells), HbF, mean corpuscular volume (MCV), hemoglobin (Hb), and markers of hemolysis: percent reticulocytes, absolute reticulocyte count (ARC), indirect bilirubin, and lactate dehydrogenase (LDH).
Conclusion
A case series of 2 patients enrolled on the IMR-SCD-102 EXT study and treated with IMR-687, showed sustained increases in F-cells/HbF, better clinical outcomes, and improved SCD biomarkers, including Hb and measures of hemolysis. These preliminary results potentially show that extended duration of treatment with IMR-687 could be beneficial to SCD patients as a monotherapy or in combination with HU.
Andemariam:Guidepoint: Honoraria; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cyclerion: Consultancy, Membership on an entity's Board of Directors or advisory committees; CRISPR/Vertex: Consultancy, Membership on an entity's Board of Directors or advisory committees; CHNCT: Consultancy; Accordant: Membership on an entity's Board of Directors or advisory committees; Imara: Research Funding; Hemanext: Membership on an entity's Board of Directors or advisory committees; Terumo BCT: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Emmaus: Membership on an entity's Board of Directors or advisory committees; Vertex: Honoraria; bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; NovoNordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Mant:Imara, Inc: Consultancy. Howard:Agios, Forma Therapeutics, Inc., Global Blood Therapeutics, Imara, Inc., Novo Nordisk, Novartis: Membership on an entity's Board of Directors or advisory committees; Imara, Inc., Novartis, Resonance Health: Honoraria. Fok:Imara, Inc: Consultancy. Hagar:Imara, Inc: Other: Data Monitoring Committee. Ballal:Imara, Inc: Current equity holder in publicly-traded company. Lufkin:Imara, Inc: Current equity holder in publicly-traded company. Lisbon:Imara, Inc: Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal